
Greenhouse gases are atmospheric gases that trap heat from the Earth’s surface, contributing to the greenhouse effect. This phenomenon is leading to significant global warming and climate change. This article aims to study in detail the types, sources, effects, and mitigation efforts related to greenhouse gases.
About GreenHouse Effect
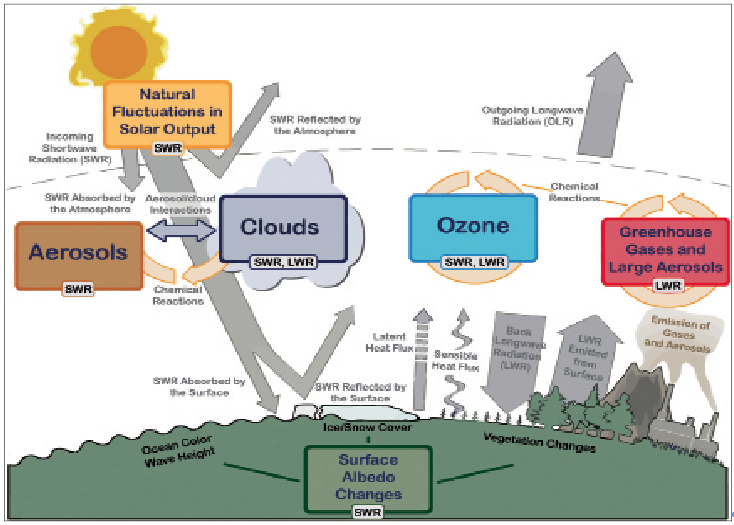
- Solar insolation (short wave radiation) reaches the Earth and is transparent to the Earth’s atmosphere.
- The greenhouse effect is absorbed by the earth’s surface, which increases its temperature. The earth’s surface radiates back the rest of the heat to the atmosphere (Long-Wave Radiation).
- Some of the heat is trapped by greenhouse gases such as Carbon dioxide, Methane, Ozone, Nitrous Oxide, Chlorofluorocarbon compounds (CFCs), and Water Vapor in the atmosphere.
- Thus, they add to the heating, which is known as the greenhouse effect of the atmosphere. This causes global warming.
- However, life on Earth could not exist without some naturally occurring greenhouse gases. Without them, no heat would be trapped in the atmosphere, leading to extreme cold or permafrost conditions.
Read our detailed article on Global Warming.
Causes of Greenhouse Effect
- The greenhouse effect is primarily caused by the accumulation of greenhouse gases in the Earth’s atmosphere, such as carbon dioxide (CO₂), methane (CH₄), nitrous oxide (N₂O), and water vapor.
- These gases trap heat from the sun, preventing it from escaping back into space, leading to a warming greenhouse effect.
- Human activities like burning fossil fuels (coal, oil, gas), deforestation, and industrial processes significantly increase the concentration of these gases causing greenhouse effect.
- Natural processes, such as volcanic eruptions and solar radiation, also contribute, but human actions are the dominant drivers of the enhanced greenhouse effect.
Greenhouse Gases
- Atmospheric gases like carbon dioxide, methane, nitrous oxide (N2O), water vapour, and chlorofluorocarbons can trap the outgoing infrared radiation from the earth’s surface, causing the greenhouse effect.
- Hence, these gases are known as greenhouse gases, and the heating effect is known as the greenhouse effect.
All these sources of greenhouse gases have been discussed in detail in the following section.
Water Vapour
- Water vapour is one of the greenhouse gases present around the planet, and it helps to reflect heat to Earth’s surface.
- Water vapour influences Earth’s climate more than other greenhouse gases.
- Water vapour is the most abundant greenhouse gas on Earth.
Carbon Dioxide
- Carbon dioxide is a meteorologically important gas as it is transparent to the incoming solar radiation but opaque to the outgoing terrestrial radiation.
- Carbon dioxide absorbs some of terrestrial radiation and reflects some part towards the earth’s surface.
- Carbon dioxide is largely responsible for the greenhouse effect.
- Its concentration is closer to the earth’s surface as it is denser than air.
Methane
- Methane is the most important greenhouse gas after carbon dioxide. It is produced from the decomposition of animal wastes and biological matter.
- Producing gobar gas (methane) from animal wastes and biological matter can restrict emissions of this gas.
Sources of Methane
- Methane is produced by decomposing animal waste or dead organisms and when plant matter is burned.
- Human activities, such as cattle ranching and decomposing waste in landfills, produce large quantities of methane.
- Methane is also released from flooded rice or paddy fields during the sowing and maturing.
- When soil is covered with water, it becomes anaerobic or lacks oxygen. Under such conditions, methane-producing bacteria and other organisms decompose organic matter in the soil to form methane (CH4).
- Millions of years ago, plants that were decomposed partially were buried underground.
- Under tectonic pressure, the carbon from these once-living organisms gets converted into methane.
- Methane is also produced by the decomposition of plants in wetlands.
- Moist and frozen soils also form methane. Moist soils that have been frozen for at least two years in a row are called ‘permafrost’.
- Permafrost exists at high latitudes and high altitudes. Specially adapted low-growing plants can live in permafrost.
- When these plants die, the carbon stored in the plant material becomes trapped in the permafrost as methane. When the permafrost melts, it releases methane into the atmosphere.
- Around 25 per cent of all methane emissions come from domesticated animals, such as dairy cows, goats, pigs, buffaloes, camels, horses, and sheep.
- These animals produce methane during the cud-chewing process. They have a special process for digesting grass and the other plant material they eat.
- Bacteria are part of this digestive process, breaking down plant matter and releasing methane as animals exhale and emit gas.
- Methane is also emitted during oil drilling, coal mining, and leaking gas pipelines (due to accidents and poor site maintenance).
Nitrous Oxide
- N2O, or Nitrous Oxide, is a greenhouse gas.
- Meanwhile, NO (nitric oxide) and NO2 (nitrogen dioxide) emissions cause global cooling through the formation of (OH) radicals that destroy methane molecules, countering the effect of GHGs.
Sources of Nitrous Oxide
- The largest human-related sources are associated with the management of soil for agriculture.
- Using nitrogen-based fertilisers to improve farm soil quality is the most significant contributor to this problem.
- Other human-related sources of nitrous oxide are sewage treatment, farm animal waste products, burning fossil fuels, and industrial production of chemicals like nitric acid.
- The chief natural sources of nitrous oxide are decomposition and bacterial action in soil.
- Rather than just using oxygen for respiration, many species of bacteria can use nitrates, nitrogen-containing molecules in the soil, as the basis for respiration.
- This process, called ‘denitrification,’ releases nitrous oxide into the atmosphere. It is also a by-product of cloud thundering.
- In addition, a large amount of nitrous oxide emission has been attributed to fertiliser application.
- This, in turn, depends on the type of fertiliser used, how and when it is used, and the tilling methods followed.
- Contributions are made by leguminous plants, such as beans and pulses, adding nitrogen to the soil.
- Incomplete combustion in internal engines is one of the major anthropogenic sources of nitrous oxides.
Recent Trends in Nitrous Oxide
- Recent studies and research show that concerns about N2O are more important than believed.
- N2O gas in the atmosphere increased during the 20th century, which continues today.
- The annual increase in atmospheric nitrous oxide has been about 0.25% each year during the last century.
- Although this seems very small, it is essential compared to other GHGs because of its potential to increase global temperatures.
- Scientific research indicates that over 100 years, nitrous oxide has a 300 times greater ‘global warming potential’ than carbon dioxide.
Limiting the N2O Production
- Agriculture is the major contributor of N2O to the atmosphere. However, the amount of N2O added to the atmosphere can be reduced by limiting the use and efficient application of Nitrogen-based fertilisers.
- Fossil fuel burning emits N2O into the atmosphere. Replacing or limiting fossil fuel use by developing an alternate energy source, such as Solar cells or electric motors, can significantly reduce N2O emissions.
- Additionally, using advanced technologies to filter out N2O from vehicular exhaust (for example, Catalytic Converters) can also be very significant in controlling N2O addition to the atmosphere.
- Industrial use of fossil-based fuel is the primary reason for industry-based N2O addition to the atmosphere. Switching to cleaner fuels and technologies can lead to reduced nitrous oxide production.
Fluorinated Gases
Fluorinated gases are artificial gases that contain fluorine. They have a lifetime of many centuries, which can significantly enhance the greenhouse effect (as they are potent greenhouse gases). There are mainly four types of fluorinated gas, they are:
- Hydrofluorocarbons (HFCs): They are used as an alternative to CFC and HCFC (ozone-depleting substances) in refrigerators and air conditioning as they have zero ozone-depleting potential.
- Other sources include the manufacture of polymer foams, fire retardants, solvents for cleaning plastic and metals, fuels, and semiconductor technology in greenhouse effect.
- Perfluorocarbons (PFCs): Perfluorocarbons are compounds majorly produced as a by-product of various industrial processes associated with aluminium production and the manufacturing of semiconductors.
- PFCs generally have long atmospheric lifetimes and high global warming potential.
- Sulphur Hexafluoride (SF6): Sulphur hexafluoride processes magnesium and manufactures semiconductors.
- It is also used as a tracer gas for leak detection.
- Nitrogen Trifluoride (NF3): NF3 is a greenhouse gas with a global warming potential (GWP) 17,200 times greater than CO2 over 100 years.
- Its chief sources are the manufacturing of semiconductors, thin film solar cells and flat-panel displays.
Black Carbon
- Black carbon (BC) is a solid particle or aerosol (though not a gas) that contributes to the warming of the atmosphere.
- Black carbon, commonly known as soot. Soot is a form of particulate air pollutant produced from incomplete combustion.
- Black carbon warms the earth by absorbing heat in the atmosphere and reducing albedo (the ability to reflect sunlight) when deposited on snow and ice.
- Black Carbon is the strongest absorber of sunlight and heats the air directly. In addition, it darkens snow packs and glaciers through deposition, which leads to ice and snow melting.
- Black Carbon disrupts cloudiness and monsoon rainfall regionally. Black carbon stays in the atmosphere for only several days to weeks.
- Thus, the effects of Black Carbon on atmospheric warming and glacier retreat disappear within months of reducing emissions.
Sources of Black Carbon
- The main sources of Black Carbon include emissions from diesel engines, cook stoves, burning open wood and biomass, forest fires, industrial processes, and power generation.
Impacts of Black Carbon
- Black Carbon absorbs sunlight and generates heat in the atmosphere, which warms the air and can affect regional cloud formation and precipitation patterns.
- When black carbon is deposited on snow and ice, it absorbs sunlight, reducing Albedo and generating more heat.
- This heats both the air above and the snow and ice below, thus resulting in the melting of glaciers and ice caps.
- Because Black Carbon remains in the atmosphere for a shorter duration (1-4 weeks), its effects are strongly regional.
- Its short lifetime also means that its climatic effects would dissipate quickly if black carbon emissions were regulated, thus directly benefiting the countries that invest in technologies and practices to reduce Black Carbon emissions.
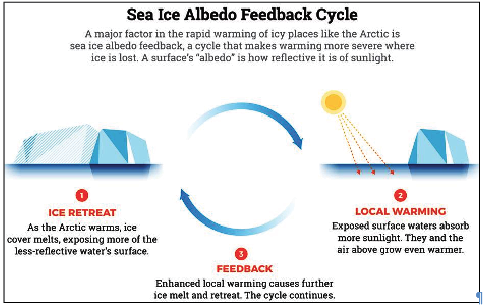
| Albedo is a measure of the reflectivity of a surface. When applied to the Earth, the albedo effect measures how much of the Sun’s energy is reflected into space. Overall, the Earth’s albedo has a cooling effect. (The term ‘albedo’ is derived from the Latin for ‘whiteness’) |
Brown Carbon
- Brown carbon aerosols are non-graphitic organic materials that are brown or yellowish.
- They come from various sources, including biomass burning, industrial emissions, fossil fuel combustion, and automobile exhaust.
- Brown carbon aerosols can also be formed in the atmosphere from reactions of biogenic organic gases and volatile organic compounds from vegetation.
- Researchers classify organic materials in the atmosphere by their ability to absorb or scatter sunlight.
- The scattering of sunlight has a cooling effect overall on the climate, while the absorption of sunlight heats the air.
- Carbon-rich materials in the atmosphere range between predominantly scattering and strongly absorbing. The compounds between the two extremes are brown carbon aerosols.
- Brown carbon aerosols are made of thousands of complex compounds, and little is known about them in detail because of their complexity.
Sources of Brown Carbon
- Large-scale wildfires in forests and grasslands release substantial amounts of brown carbon.
- Burning crop residues and other agricultural waste contributes to brown carbon emissions.
- Diesel engines, in particular, emit brown carbon particles due to incomplete combustion.
- Combustion in industrial settings, including power plants, can produce brown carbon.
- Burning wood, coal, and other materials in stoves and open fires to heat and cook releases brown carbon.
- Decomposition of organic waste in landfills can produce brown carbon aerosols.
Impacts of Brown Carbon
- Most aerosols reflect sunlight, and some absorb it. Many of these nanoparticles cause severe health effects in addition to climate effects.
- Brown carbon is believed to absorb heat strongly in ultraviolet and less significantly in visible wavelengths.
Effects of Greenhouse Gases
Greenhouse gases, such as carbon dioxide, methane, and nitrous oxide, trap heat in the Earth’s atmosphere, leading to global warming. This global warming has far-reaching consequences, including:
- Rising sea levels: As glaciers and ice sheets melt, sea levels rise, threatening coastal cities and islands.
- Extreme weather events: Due to climate change, more frequent and intense hurricanes, droughts, floods, and heat waves are becoming common.
- Ocean acidification: Increased atmospheric carbon dioxide dissolves into the oceans, making them more acidic and harming marine ecosystems.
- Biodiversity loss: Climate change is disrupting ecosystems, leading to habitat loss and species extinction.
- Agricultural impacts: Changes in temperature and precipitation patterns can affect crop yields, leading to food shortages and price fluctuations.
- Health risks: Climate change is linked to heat-related illnesses, respiratory problems, and the spread of diseases.
Global Efforts to Reduce Greenhouse Gas Emissions
- The Paris Agreement is a global pact that aims to limit global warming to well below 2°C above pre-industrial levels, with efforts to limit it to 1.5°C.
- To reduce energy consumption, improve energy efficiency, and adopt sustainable practices.
- To invest in projects that reduce or capture carbon emissions.
- To adopt solar, wind, hydro, and geothermal energy sources to reduce reliance on fossil fuels.
- To implement carbon pricing, emissions trading systems, and regulations to encourage low-carbon technologies.
Conclusion
Greenhouse gases are essential for Earth’s climate but are causing severe warming due to human activities. Addressing this issue involves global cooperation to reduce emissions and implement sustainable practices to mitigate climate change and protect the environment for future generations.
Greenhouse Effect and Global Warming
- The greenhouse effect is a natural process where greenhouse gases like carbon dioxide (CO₂), methane (CH₄), and water vapor trap heat in the Earth’s atmosphere, keeping the planet warm enough to support life.
- However, human activities such as burning fossil fuels and deforestation have increased the concentration of these gases, intensifying the greenhouse effect and leading to global warming.
- Global warming refers to the long-term rise in Earth’s average temperature, causing climate change, melting ice caps, rising sea levels, and extreme weather events.
Greenhouse Gas Protocol (GHG Protocol)
- The Greenhouse Gas Protocol (GHG Protocol) is the most widely used international framework for measuring and managing greenhouse gas (GHG) emissions.
- It was developed by the World Resources Institute (WRI) and the World Business Council for Sustainable Development (WBCSD) to provide businesses, governments, and organisations with standardised methods for quantifying and reporting GHG emissions.
- The GHG Protocol consists of three main scopes:
- Scope 1: Direct emissions from sources owned or controlled by the company, such as emissions from fuel combustion.
- Scope 2: Indirect emissions from the generation of purchased electricity, steam, heating, and cooling consumed by the company.
- Scope 3: All other indirect emissions that occur in a company’s value chain, including both upstream and downstream emissions like transportation, waste disposal, and product use.
Frequently Asked Questions (FAQs)
What is Greenhouse effect?
The greenhouse effect is the process by which certain gases in the Earth’s atmosphere trap heat from the Sun, preventing it from escaping back into space and keeping the planet warm enough to support life.
What Causes the Greenhouse effect?
The greenhouse effect is caused by the accumulation of greenhouse gases like carbon dioxide (CO₂), methane (CH₄), and water vapor in the atmosphere, which trap heat and cause the Earth’s temperature to rise.
Which gas is responsible for the Greenhouse effect?
Carbon dioxide (CO₂) is one of the primary gases responsible for the greenhouse effect.






