Acid Rain is a significant environmental issue that has garnered attention for its detrimental impacts on ecosystems, human health, and infrastructure. As a key topic in environmental science and policy discussions, understanding it is crucial for environmental conservation and sustainable development. This article aims to study acid rain in detail, including its meaning, causes, processes involved, impacts, and related concepts such as acid deposition.
What is Acid Rain?
- Any precipitation in the form of rain, fog, mist, or snow that is acidified is termed Acid Rain.
- This acidity is primarily caused by the presence of Sulfur dioxide (SO₂) and Nitrogen Oxides (NOx) in the atmosphere, which react with water vapour to form Sulfuric acid (H₂SO₄) and Nitric acid (HNO₃), respectively.
- These acids then fall to the ground with the precipitation, leading to environmental and structural damage.
Rain is naturally acidic, but acid gases make it even more acidic. Acid rain has a pH value of less than 7 on the pH scale.
Mechanism of Acid Rain
- Air pollution from burning fossil fuels increases the concentration of oxides of Sulphur and Nitrogen in the air.
- When these oxides react with the moisture in the air, it leads to acid rain.
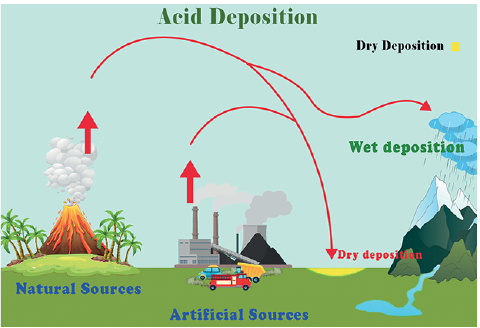
Sources of Acid Rain
Acid rain results from polluting gases like Sulphur dioxide, Nitrogen oxides, Formic acid, Carbon dioxide, Carbon monoxide, Methane, and Chlorine, as well as Phosphoric acid and hydrochloric acid.
| COMPOUNDS | NATURAL SOURCES | ANTHROPOGENIC SOURCES |
|---|---|---|
| Sulphur Dioxide | Volcanoes, Oceans, Decomposition of organic matter | Fossil fuel burning, Industrial processes, Thermal power plants based on coal. |
| Nitrogen Oxides | Volcanoes, Lightening, Decomposition of organic matter, Forest fires | Fossil fuel burning, Power plants based on coal, Biomass burning |
| Formic Acid | Forest fires | Biomass burning |
| Carbon Dioxide | Respiration, Decomposition | Fossil fuel burning, Industrial processes |
| Carbon Monoxide | Isoprene emissions by plants | Biomass burning, Industrial sources |
Chemistry of Acid Rain
- As a result of both natural and anthropogenic activities, the concentration of sulphur and nitrogen increases in the air.
- These are present in the air as Sulphur dioxide and Nitrogen dioxide.
- In drier regions, some of this is deposited in the form of dry deposits.
- Both sulphur dioxide and nitrogen dioxide are acidic oxides and react with water to form acids.
- Sulphur dioxide reacts with water to form sulphurous acid.
- Substances in the upper atmosphere then catalyse the reaction between sulphurous acid and oxygen to form sulphuric acid.
- Similarly, nitrogen dioxide reacts with water to form a mixture of nitric acid and nitrous acid.
- Sulphur dioxide reacts with water to form sulphurous acid.
- Both sulphuric acid and nitric acid are soluble in water and are the major acids present in acid rain. They come down as Acid Rain.
Causes of Acid Rain
- Acid rain is primarily caused by releasing sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) into the atmosphere, which reacts with water vapour and other chemicals to form sulfuric and nitric acids.
- These pollutants are mainly produced by burning fossil fuels in power plants, factories, and vehicles.
- Volcanic eruptions and wildfires can also contribute to the release of these gases.
- Once formed, acid rain falls to the ground, damaging ecosystems, soil, water bodies, and buildings and posing risks to human health and the environment.
Types of Acid Deposition
The types of acid deposition in the air depend upon the moisture content in the atmosphere. Based on the moisture content, acid deposition can be classified as:
Wet Deposition
- Acidic deposition is in the regions where atmospheric conditions are wet.
- These acidic elements in the air fall to the ground in precipitations like rain, fog, mist, or snow.
- Wet deposition forms, such as acid rain, are common in regions with high moisture levels.
- This type of acid deposition is common in the east margins of tropical regions, west margins of temperate regions, and polar regions of the earth, with a high concentration of pollution sources.
Dry Deposition
- Acidic deposition in the regions where atmospheric conditions are dry.
- These acidic elements in the air fall to the ground with the wind and are mixed with aerosols.
- For example, dust particles, smoke, pollen grains, salt particles, etc, act as the locus for acid deposition in dry areas.
- These acidic elements settle on the ground along with aerosols, which are then washed out by the rain during the rainy season.
- This type of acid deposition is common in the west margins of Tropical Areas and the east margins of Temperate areas where there is a concentration of pollution sources.
Areas Affected by Acid Rain
- A high concentration of pollutants, which is common in industrial areas, is necessary for acid rain to occur.
- Thus, Acid Rain commonly occurs in areas near industrial regions.
- For example, in India, acid rain was first reported in 1974 in the Mumbai region, which has a high concentration of industries.
- However, it is not exactly limited to industrial regions only.
- When acid gases are released, they rise to the sky and are carried by strong winds.
- Thus, Acid Rain is a problem all over the world.
- For example, Acid rain in Scandinavian countries is caused by air pollution in Britain and other European countries.
Impacts of Acid Rain
The multi-faceted impacts of Acid Rain can be seen as follows:
- On Soil
- Leaching occurs when acid rain deposition adds hydrogen ions to the soil.
- This displacement of important nutrients like calcium, magnesium, and potassium pushes the nutrients deeper into the soil, making it difficult for plant roots to get them and making the soil infertile.
- It affects respiration by the soil organisms.
- As the nutrients become scarce in the soil, it also impacts the decomposition rate.
- If acid rain falls on alkaline soil, the acid becomes neutral, so the plants are not hugely affected. However, if the soil is slightly acidic, it can be disastrous.
- On Vegetation
- Acid rain reduces the photosynthesis rate in plants. This affects their growth rate.
- The abnormal growth rate has various impacts, such as discolouration, a decline in biomass, early ageing, and tree death.
- Moreover, the absorption of acidic ions from the soil affects plant metabolism.
- On Microorganisms
- Microorganisms have a very limited range of pH in which they can survive. Any change in this pH affects their number in any environment. As most microorganisms, like bacteria and protozoa, except fungi, thrive in an environment with near-neutral pH, acid rain has a severe impact on the population of microorganisms.
- Acid rain changes the area from bacteria-dominated to fungi-dominated. This increases fungal diseases and reduces the decomposition rate of organic material in the soil.
- On Aquatic Life
- The pH value is an important determinant of metabolic processes in aquatic regions. The eggs or sperms of fish, frogs and other aquatic organisms are very sensitive to pH change. Acid rain kills their gametes, affecting their life cycles and productivity.
- This, in turn, impacts the food chain in the aquatic environment, causing ecological imbalance. Further, acid rain kills plankton, a primary producer in the aquatic food chain. This makes water bodies lifeless, as fish die because of the lack of food. This impacts the livelihood of fishermen.
- On Terrestrial Life
- Acid rain affects the photosynthesis rate in plants. Plants are the primary food source in terrestrial ecosystems, affecting the food chain. Thus, there is a threat to the population of terrestrial animals because of acid rain, but this is an indirect threat.
- On Humans
- Acid rain looks, feels, and tastes just like clean rain. Its harm to people is not direct, however, as it does not have an acidic pH to burn human skin.
- The air pollution that causes acid rain is more damaging to human health. Sulphur dioxide and nitrogen oxides, the major sources of acid rain, can irritate or even damage our lungs, reduce visibility, and cause irritation to the skin, eyes, and respiratory tract.
- Moreover, sulphur dioxide particles in the air can cause chronic lung problems like asthma and bronchitis. Additionally, the nitrogen oxides that create acid rain promote the formation of ground-level ozone, which is harmful.
- Further, acid rain impacts water resources and food chains, of which humans are part. Thus, acid rain can cause food poisoning.
- On Buildings and Monuments
- Many old, historic, ancient buildings and works of art/textiles, etc., are adversely affected by acid rain. Acid rain destroys limestone and marble. Smoke and soot cover such objects. Acid fumes in the air slowly dissolve/flake away the surfaces. Many buildings/monuments, such as the Taj Mahal in Agra, have suffered from acid rain.
Measures to Control Acid Rain
Any measure that controls the emission of pollutants like sulphur and nitrogen oxides will help reduce acid rain. Accordingly, the following are some of the control measures:
- Cleaner Fuels: Use low sulphur fuel, natural gas or washed coal in thermal power plants.
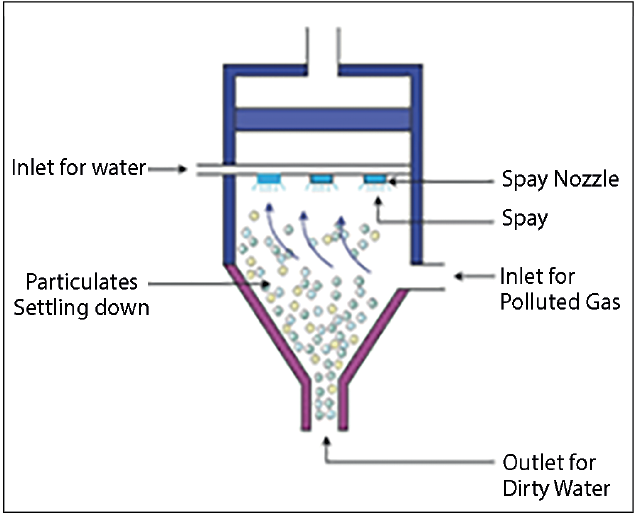
- Scrubbers: In power plants, attaching devices known as ‘scrubbers’ are used in the chimneys.
- These scrubbers reduce the sulphur produced in the smoke by 90 – 95%.
- Catalytic Converters: Fitting catalytic converters into vehicles’ exhaust pipes also reduces the amount of sulphur dioxide produced by the vehicles.
- Shift to Renewable Energy: Alternate energy sources like tidal, wind, hydropower, etc, can reduce the emission of harmful gases.
- Other Measures: Other measures to control Acid Rain can include:
- Add neutralising agents like lime to increase the pH value of the water.
- Shift towards better emission standards.
- Industries must regularly inspect and clean all their emission equipment, chimneys and pipes.
- A widespread and nationwide effort must be made to make people aware.
Conclusion
Acid rain is a complex environmental issue with far-reaching effects on ecosystems, infrastructure, and human health. It can become an environmental hazard or disaster in the industrialised world, and hence, strong measures must be taken to prevent it. Addressing this challenge requires a comprehensive approach involving regulation, technological innovation, international collaboration, and public engagement.
Frequently Asked Questions (FAQs)
What is Acid Rain?
Acid rain refers to precipitation (rain, snow, sleet, or fog) with higher levels of sulfuric and nitric acids, making it more acidic than normal rainwater. It results from the release of pollutants into the atmosphere.
How is acid rain formed?
Acid rain forms when sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) are emitted into the atmosphere, where they react with water, oxygen, and other chemicals to create sulfuric and nitric acids.
What causes acid rain?
Acid rain is caused mainly by the burning of fossil fuels in power plants, vehicles, and industries, releasing SO₂ and NOₓ into the atmosphere.
What are the effects of acid rain?
Acid rain harms ecosystems by acidifying water bodies, damaging soil, and weakening trees. It also erodes buildings, monuments, and infrastructure and negatively affects human health by polluting air and water.
How to control acid rain?
Acid rain can be controlled by reducing emissions of SO₂ and NOₓ through cleaner energy sources, stricter pollution regulations, adopting renewable energy, and using technology like scrubbers in power plants.