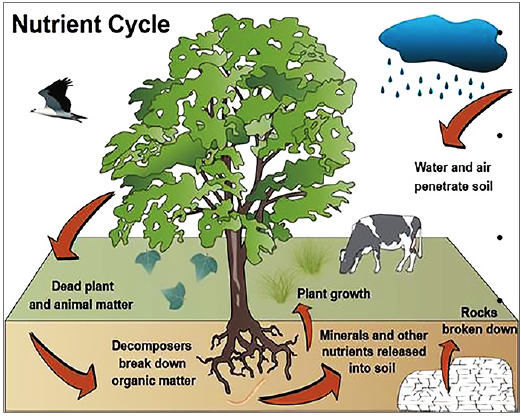
Nutrient Cycle, also known as the Biogeochemical Cycle, ensures the continuous availability of nutrients necessary for life processes. By contributing to ecosystem stability and productivity, these cycles play a significant role in sustainable growth and ecological balance. This article aims to study in detail the concept of the nutrient cycle, its meaning, types and some important nutrient cycles such as the Carbon Cycle, Nitrogen Cycle, Water Cycle (Hydrological Cycle), Phosphorus Cycle and Sulphur Cycle.
What is Nutrient Cycle?
- Nutrient Cycle, also known as Biogeochemical Cycle, is the process by which essential elements and compounds move through the Earth’s ecosystems, connecting the biotic (living) and abiotic (non-living) components.
- These cycles ensure the continuous availability of nutrients required for the growth, survival, and reproduction of organisms.
Types of Nutrient Cycles
Based on the reservoir in which the nutrient cycling is taking place, there are two types of nutrient cycles:
Gaseous Nutrient Cycle
For this cycle, the reservoir is the atmosphere. Important gaseous cycles include Carbon, Nitrogen and Water.
Sedimentary Nutrient Cycle
For this cycle, the reservoir is the Earth’s crust. Important Sedimentary cycles include Phosphorous and Sulphur.
Important Nutrient Cycles
Some of the important biogeochemical cycles in an ecosystem include:
- Carbon Cycle
- Nitrogen Cycle
- Water Cycle or Hydrological Cycle
- Phosphorous Cycle, and
- Sulphur Cycle
Each of these cycles has been discussed in detail in the sections that follow.
Carbon Cycle
- Carbon is one of the essential parts of living organisms as it constitutes 49 percent of dry weight of the organisms.
- As for sources of Carbon, 71 per cent of carbon is dissolved in oceans.
- It is also present in the earth’s crust as a fossil fuel.
- Carbon is present in the atmosphere in mainly two forms, Carbon dioxide and methane.
- Thus, the cycle involves all the components, including the atmosphere, oceans, living organisms and dead matter.
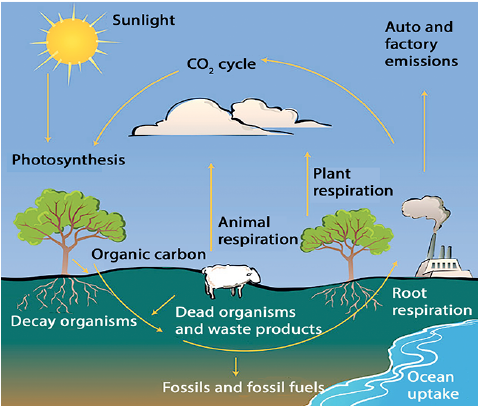
Processes Involved in Carbon Cycle
The cycle is completed in following steps:
Photosynthesis
- It is the process in which green plant, in the presence of sunlight, converts atmospheric inorganic carbon (carbon dioxide) into organic matter (food) and release oxygen.
- This food is utilised by the plants for their own functions and also passed on to the organisms of higher trophic levels.
Respiration
- The food needs to be converted into energy.
- Respiration is the process in which this food is oxidised and converted to energy, releasing carbon dioxide and water.
- This Carbon dioxide (CO2) is released into the atmosphere.
Decomposition
- Only a part of the photosynthesised food is oxidised to produce energy.
- The remaining part gets stored as biomass of an organism which is acted upon by the detritus on the death of the organism, releasing the carbon dioxide in the atmosphere again.
Combustion
- Other than decomposition, the burning of biomass directly releases Carbon dioxide into the atmosphere.
Impact of Human Activities on Carbon Cycle
- The natural balance of the cycle of carbon has been distorted by the human activities, especially since the industrialization.
- Destruction of forests combined with the release of carbon dioxide from industries and power plants have led to increasing Carbon dioxide emissions, which is causing global warming.
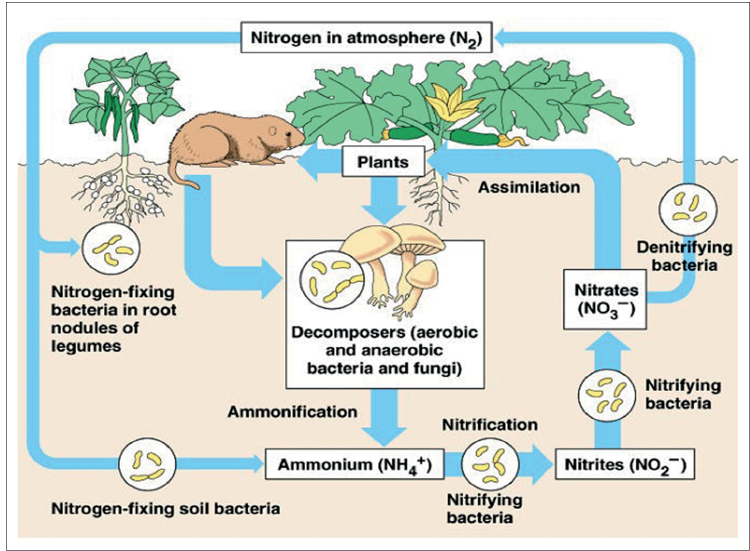
Nitrogen Cycle
- Nitrogen Cycle is a biogeochemical process that transforms nitrogen gas from the atmosphere into forms that are usable by living organisms, and then back into the atmosphere.
- The cycle ensures a continuous supply of nitrogen for plant growth, which supports the entire food chain.
Processes Involved in Nitrogen Cycle
The Cycle of Nitrogen involves five main processes:
Nitrogen Fixation
- The nitrogen fixation process involves the conversion of gaseous Nitrogen into a form that plants can easily absorb like ammonia, nitrates, etc.
- As Nitrogen gas is an inert gas, it requires a substantial amount of energy to break its atoms apart so that they can combine with other atoms.
- Atmospheric Nitrogen can be fixed mainly through three processes:
Atmospheric Fixation
- In this type of fixation, the energy is provided by activities like lightning, combustion or volcanic activity, which helps in the fixation of nitrogen.
- This energy breaks the otherwise inert nitrogen gas.
- Ultimately, the nitrogen molecule reacts with oxygen forming oxides of nitrogen.
- It is converted into nitrates when it gets dissolved in the rain and carried down to earth.
Industrial Fixation
- Artificially, nitrogen fixation can be done in industries under a specific condition that involves high temperature and high pressure.
- Through this process, atmospheric Nitrogen gas and Hydrogen gas are combined to produce Ammonia which is further processed to make Urea and thus passed on to the Plants.
Bacterial Fixation
This ability to fix nitrogen is found only in some bacteria:
- Symbiotic Bacteria: e.g. Rhizobium in the root nodules of leguminous plants.
- Free-living or Non-symbiotic Bacteria: e.g. Nostoc, Azobacter, Cyanobacteria can combine atmospheric or dissolved nitrogen with hydrogen to form ammonia.
Nitrification
- Though Plants can take up Nitrogen in the form of Ammonia, most of the ammonia is converted into nitrites or nitrates by Nitrosomonas and Nitrobacter bacteria, respectively.
- The plants then take up this nitrate.
- In the first step, Nitrosomonas bacteria convert Ammonia to nitrites. In the second step, Nitrobacter oxidises the nitrites to nitrates.
Assimilation
- These nitrates are, in turn, taken up by the plants through their roots which go on to make organic molecules, including proteins, RNA, DNA etc.
- These are the essential part of plant and animal tissues.
Ammonification
- This is part of the decaying process.
- Under this process, the nitrogen taken up by the organism is again converted back to inorganic ammonia.
- For this, ammonifying bacteria helps in converting waste products like urea and uric acids and also the dead remains of the organisms into ammonia.
Denitrification
- This is the process by which nitrogen gas is liberated from ammonia and released back into the atmosphere.
- For this process, denitrifying bacteria, living deep in the soil helps.
- Thus, through this cycle, Nitrogen is again replenished back into the atmosphere, and the cycle is complete.
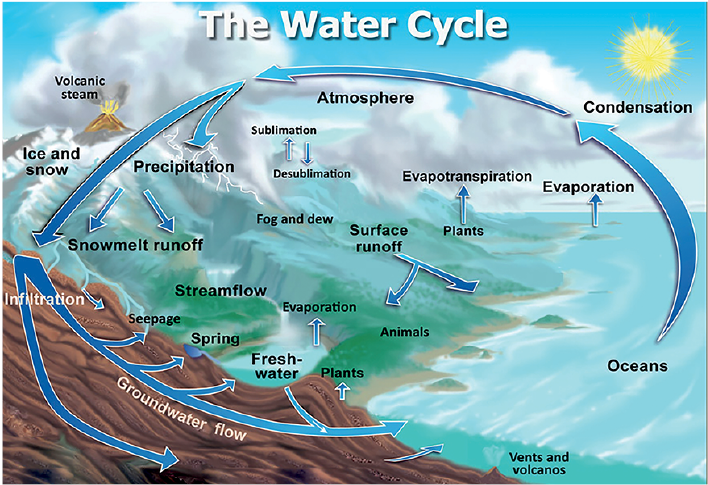
Water Cycle or Hydrological Cycle
- The water cycle, also known as the hydrological cycle, is the continuous movement of water within the Earth and its atmosphere.
- It describes how water circulates through various phases and reservoirs, including the atmosphere, oceans, rivers, lakes, groundwater, and glaciers.
- This cycle is crucial for sustaining life, regulating climate, and supporting ecological processes.
- This cycle is mainly driven by two forces: Solar Radiation and Gravity.
Processes Involved in Water Cycle
The Cycle of Water involves the following processes:
Evapotranspiration
- Due to solar radiation, water from oceans, rivers, lakes, ponds etc evaporates into the atmosphere.
- Water is evaporated from the plants leaves also in the process of transpiration.
- This water remains in the atmosphere in vapor state and forms cloud which can be carried to faraway places with winds.
Condensation
- As the water vapor is much lighter than the surrounding air, it rises up and form clouds.
- These clouds again rise upwards which involves the release of heat of condensation, warming the cloud more and thus making it lighter.
- After rising to great heights, these clouds start to condense and changes into tiny water droplets.
Precipitation
- These tiny droplets further combine to form big water droplets which fall on the surface of earth in the form of rain, snow, hail and sleet under the force of gravity.
Phosphorous Cycle
- The phosphorus cycle is the biogeochemical process that describes the movement of phosphorus through the Earth’s lithosphere (rock), hydrosphere (water), and biosphere (living organisms).
- Unlike other biogeochemical cycles, the phosphorus cycle does not involve a significant atmospheric component.
- Instead, phosphorus primarily moves through soil, water, and organisms.
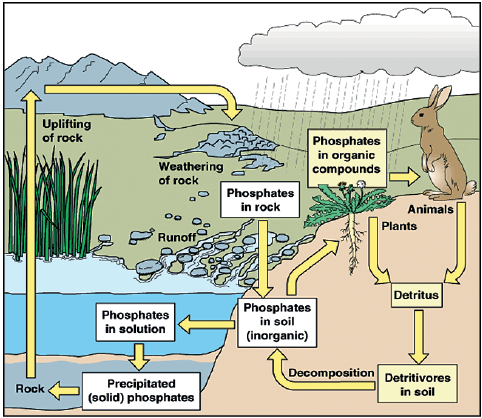
Processes Involved in Phosphorous Cycle
The Phosphorus Cycle involves the following processes:
Weathering
- Phosphorus is mainly found in rocks in the form of Phosphates.
- This is released from the rocks by weathering, especially during the rainy season.
- Due to this, the inorganic Phosphate ions are distributed through soil and water.
Circulation Through Trophic Levels
- Plants absorb these inorganic phosphate ions through their roots from water and soil.
- Animals at higher trophic levels receive the phosphorus by eating the plants or the plant-eating animals.
- In plants and animals, this phosphorus is incorporated into organic molecules including DNA etc.
Decomposition
- Finally, the phosphate-solubilizing bacteria liberates the phosphorus into the soil by decomposing the dead organisms and other waste products.
Sulphur Cycle
- The Sulfur Cycle is the biogeochemical process that describes the movement of sulfur through the Earth’s atmosphere, lithosphere (rock), hydrosphere (water), and biosphere (living organisms).
- This cycle is crucial for various biological and chemical processes and plays a significant role in regulating the Earth’s climate and soil fertility.
- Sulphur is one of the important components of proteins, amino acids, enzymes and vitamins.
- Thus it is essential for both plants and animals.
Processes Involved in Sulphur Cycle
The Sulphur Cycle involves the following steps:
Weathering
- Weathering of sulphur-containing rocks releases the sulphur into the soil, which gets converted into sulfate when it comes into contact with air.
Precipitation
- Hydrogen sulfide and sulphur dioxide are released into the atmosphere through volcanic eruptions and the burning of fossil fuels.
- The gases react with tiny water droplets in the atmosphere, forming sulphuric acid which falls to the earth in the form of precipitation, thus mixing with the soil.
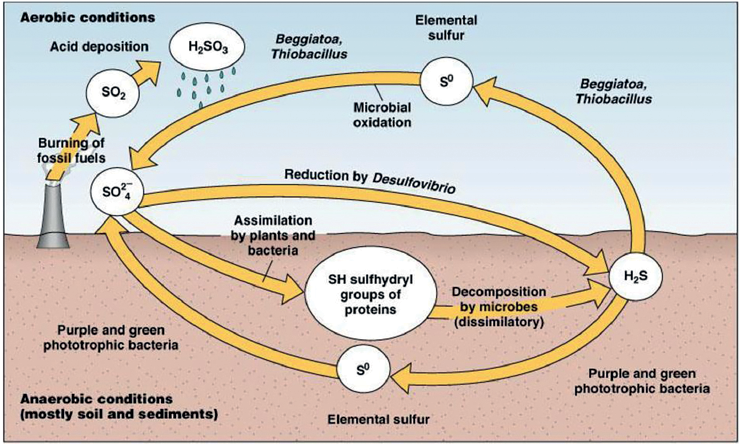
Circulation Through Trophic Levels
- Plants receive sulphur by absorbing the ions of the sulfate salts from the soil.
- All the other animals get their requirement of sulphur by eating the plants or the plant-eating animals.
Decomposition
- Finally, the sulfate salts are released back to the soil by decomposition of the organism after their death which can again be taken up by the plants.
- Through surface runoff or otherwise, the remaining sulphur settles down in the ocean after combining with iron, forming ferrous sulfide.
Importance of Nutrient Cycles
- Sustains life: Ensures a continuous supply of essential nutrients for organisms.
- Maintains ecosystem balance: Helps regulate the amount of nutrients in the environment, preventing imbalances.
- Supports food production: These biogeochemical cycles contribute to fertile soil, which is essential for agriculture.
Conclusion
Nutrient Cycles are fundamental to life on Earth. Understanding these complex processes is crucial for addressing environmental challenges and building a sustainable future. By adopting responsible practices, we can protect and restore the delicate balance of these biogeochemical cycles for generations to come.
Frequently Asked Questions (FAQs)
How does deforestation affect the Nutrient Cycle?
Deforestation affects the Nutrient Cycle in several ways, such as soil erosion and consequent nutrient loss, altered hydrological cycle, food chain disruption, etc.
How is soil important in the nutrient cycle?
Soil plays an important role in the nutrient cycle by acting as a reservoir, storing, transforming, and releasing nutrients essential for life.
Why is nutrient cycling important?
By moving nutrients from environment to organisms and back again, these cycles play crucial roles in food production, sustaining life, maintaining ecological balance, etc.
What is the nutrition cycle’s primary function?
The primary function of the nutrient cycle is to continuously recycle essential elements within an ecosystem.