
Ozone Layer Depletion has emerged as one of the most prominent environmental concerns of the present time. Ozone Layer Depletion has led to concerns about increased UV exposure and its consequent effects on health, ecosystems, and climate. This article aims to study in detail Ozone Layer Depletion, its major causes, effects, and measures taken at the India and international levels, including the Vienna Convention, Montreal Protocol and Kigali Agreement.
What is Ozone Layer?
- The Ozone Layer describes the protective layer of naturally occurring gas found about 10-50 km above the earth’s surface. It protects us from harmful ultraviolet radiation.
- Ninety percent of the ozone in the atmosphere is in the Stratosphere, and 10 percent is in the Troposphere.
- The ozone in the stratosphere is commonly known as the ozone layer.
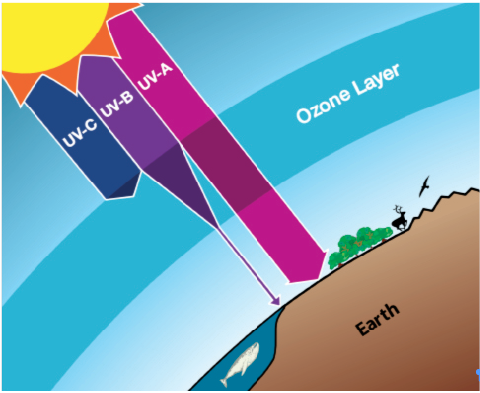
- The total amount of Ozone in the Ozone Layer varies with location on the time scales that range from daily to seasonal.
- Stratospheric winds, chemical production, and ozone destruction cause the variations.
- Because of the seasonal wind patterns in the stratosphere, total ozone is generally lowest at the equator and highest near the poles.
| The thickness of the ozone in a column of air from the ground to the top of the atmosphere is measured in terms of Dobson Units (DU). |
| Ozone – Ozone is a naturally occurring gas present in the atmosphere. – Chemically, it is a three-atom allotrope of oxygen denoted as O3. – Ozone is created when the Oxygen we breathe (O2) is split by sunlight into single oxygen atoms, which then join with O2 molecules to make ozone (O3). |
What is Ozone Layer Depletion?
- Ozone Layer Depletion refers to the thinning the Ozone layer in the Earth’s Stratosphere.
- Ozone layer depletion is a thermodynamically unstable gas that readily decomposes into molecular oxygen.
- Normally, a dynamic equilibrium exists between the production and decomposition of ozone molecules.
- Ozone layer depletion occurs when this balance of stratospheric ozone is tipped in favour of destruction.
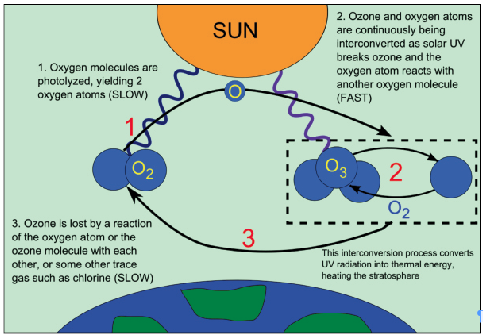
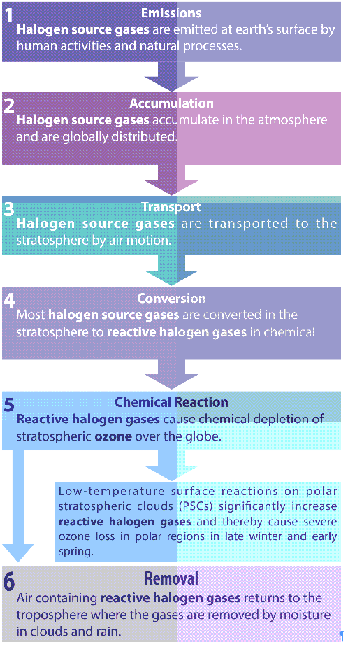
Mechanism of Ozone Layer Depletion
- Ozone Layer is destroyed when it reacts with nitrogen, hydrogen, chlorine, or bromine molecules.
- Some molecules that destroy the ozone layer occur naturally, while others have anthropogenic origins.
- Although natural phenomena can cause temporary ozone loss, chlorine and bromine released from synthetic compounds are now accepted as the main cause of a net loss of stratospheric ozone in many parts of the world.
- Most of these gases accumulate in the lower atmosphere because they are unreactive and do not dissolve readily in rain or snow.
- Eventually, the ozone layer is transported to the stratosphere, where it is converted to more reactive gases that participate in reactions that destroy ozone.
Causes of Ozone Depletion
Ozone Layer Depletion occurs because of natural as well as man-made causes:
- Natural Causes: Several naturally occurring substances destroy ozone layer. These are Hydrogen oxide (HOx), Methane (CH4), Hydrogen gas (H2), Nitrogen oxides (NOx), Chlorine monoxide (ClO), stratospheric aerosols and gases from volcanic eruptions.
- Man-made Causes: Certain industrial processes and consumer products emit halogen source gases into the atmosphere, depleting the ozone layer.
Ozone Depleting Substances (ODSs)
Some of the most common Ozone Layer Depleting Substances (ODSs) are listed below:
Chlorofluorocarbons (CFCs)
- Chlorofluorocarbons (CFCs) are non-reactive, non-flammable, non-toxic organic molecules. They are therefore used in refrigerators and air conditioners, in the production of plastic foam, and by the electronic industry for cleaning computer parts.
- The most common Chlorofluorocarbons (CFCs) are CFC-11, CFC-12, CFC-113, CFC-114, and CFC-115.
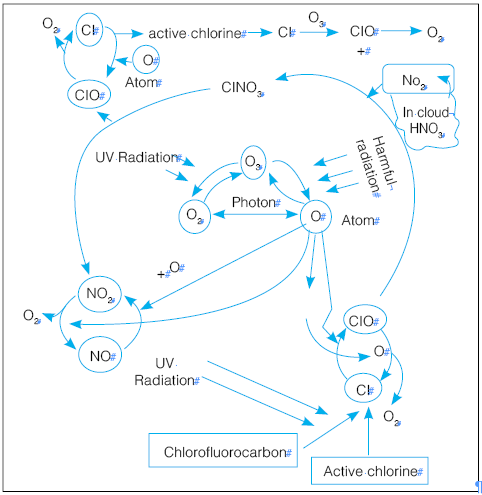
- CFCs transport agents that continuously generate chlorine radicals into the stratosphere and damage the ozone layer.
- One free Chlorine atom from a CFC molecule can do much damage, destroying ozone molecules for a long time.
- CFCs have a lifetime in the atmosphere of about 20 to 100 years.
Hydrochlorofluorocarbons (HCFCs)
- Hydrochlorofluorocarbons (HCFCs) are used as substitutes for CFCs because many of their properties are similar. They are also less harmful to ozone because they have a shorter half-life and release fewer Chlorine atoms.
- But as they remain harmful to the ozone layer, they are considered only a temporary solution and their use has been banned in developed countries since 1930.
- They are used as refrigerants (refrigerators, freezers, and air conditioning systems) and insulative foams.
Nitrogen Oxides (NOx)
- As CFCs are being phased out through international agreements, Nitrous oxide has become the largest ozone-depleting substance emitted by human activities and is expected to remain the largest throughout the 21st century.
- In addition to soil fertilisation, nitrous oxide is emitted from livestock manure, sewage treatment, combustion and certain other industrial processes.
- Dentists use it as a sedative (so-called “laughing gas”).
- In nature, soil and ocean bacteria break down nitrogen-containing compounds, releasing nitrous oxide.
- About one-third of global nitrous oxide emissions are from human activities, while the rest are emitted naturally.
- Nitrous oxide, like CFCs, is stable when emitted at ground level but breaks down through photolysis when it reaches the stratosphere to form other gases, called nitrogen oxides, that trigger ozone-destroying reactions.
- The global lifetime of N2O is approximately 114 years.
Halons
- They are compounds formed by Br, F and C.
- Because of their ability to put out fires, they are used in fire extinguishers, although their manufacture and uses are prohibited in many countries because of their ozone-depleting action.
- Their ability to harm the ozone layer depletion is very high because they contain Bromine, which is much more effective than atom destroying ozone than Chlorine. I
- It has been used historically as a fire suppression agent and for fighting fires, but it is now only allowed in minimal situations.
Methyl Bromide (CH3Br)
- It is a very effective pesticide used to fumigate soils and many crops.
- Given its content in Bromine, it damages the ozone layer depletion and has a high ozone depletion potential.
- Many countries are taking steps to ban or regulate Methyl Bromide.
- It is historically used in fumigation, soil treatment, pest control, quarantine, and market gardening.
Carbon Tetrachloride (CCl4)
- It is a compound widely used as a raw material in many industries, such as manufacturing CFCs and as a solvent.
- It was no longer used as a solvent when it was found to be carcinogenic.
- It is also used as a catalyst in certain processes where chlorine ions need to be released.
- It is used in fire extinguishers and as a fumigant to kill insects in grain.
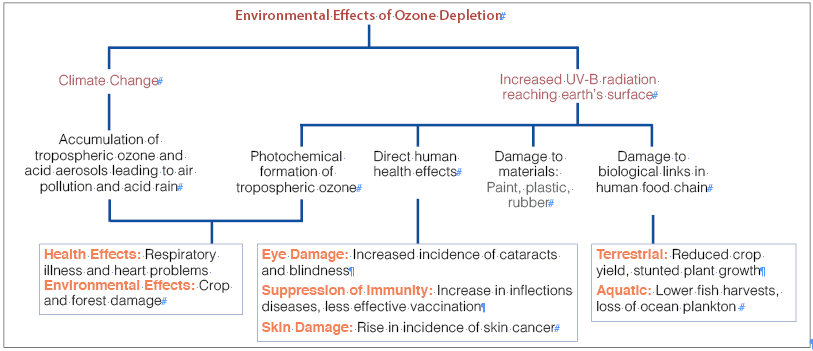
Effects of Ozone Layer Depletion
A small dose of UV-B radiation is useful as it promotes vitamin D synthesis in living organisms. UV radiation also acts as a germicide that controls microorganisms. However, with the depletion of the ozone layer, the atmospheric shield from the UV rays weakens as it allows harmful rays to reach the earth’s surface. Some of the effects of ozone layer depletion are as follows:
- On Human Beings
- Increased risk for developing several skin cancers, skin rashes and skin ageing. Malignant melanoma and basal and squamous cell carcinoma are the most common cancers.
- Leads to difficulty in breathing, chest pain, and throat irritation.
- A suppressed immune system may lead to severe infectious diseases.
- UV rays are harmful to our eyes. Direct exposure to UV rays can lead to Cataract problems, Photokeratitis or snow blindness.
- Prolonged exposure may permanently damage the cornea.
- It can lead to DNA mutation.
- On Plants
- It affects plant growth by altering the physiological and developmental processes of the plants as it inhibits photosynthesis.
- Causes mutation.
- Alters the Biodiversity in different ecosystems.
- Affects plant competitive balance, plant pathogens and biogeochemical cycles.
- On Aquatic Ecosystem
- Affects the productivity of marine/freshwater organisms.
- Affects the distribution of phytoplankton, which forms the foundation of aquatic food webs.
- Damages the early development stages of fish, shrimp, crabs, amphibians and other animals.
- Affects reproductive capacity and impairs larval development.
- On Bio-Geochemical Cycles
- Affects terrestrial and aquatic bio-geo-chemical cycles, thus altering sources and sinks of greenhouse gases.
- Alters the delicate balance among different ecosystems.
- Changes in the production and decomposition of plant matter.
- Reduction of primary production changes in the uptake and release of important atmospheric gases.
- Reduction of plankton growth in the upper ocean.
- Increased degradation of aquatic dissolved organic matter.
- On Non-Living Materials
- Accelerates the photodegradation rates of synthetic polymers, naturally occurring biopolymers and some other materials of commercial interest, thus limiting their lifetime.
- Affects the quality of these materials, ranging from discolouration to loss of mechanical integrity.
Global Efforts to Control Ozone Layer Depletion
Some of the major efforts taken at the global level to control Ozone Layer Depletion are discussed below:
Vienna Convention for the Protection of the Ozone Layer
- The Vienna Convention for the Protection of the Ozone Layer is a legally binding agreement that aims to protect the ozone layer.
- The Vienna Convention was the first convention of any kind to be signed by every country involved, taking effect in 1988.
- The Vienna Convention did not require countries to take concrete actions to control ozone-depleting substances. Instead, a provision was added that Protocols to control these substances would be adopted if and when warranted.
Montreal Protocol on Substances that Deplete the Ozone Layer
- The Montreal Protocol on Substances that Deplete the Ozone Layer is a global agreement to protect the Earth’s ozone layer by phasing out the chemicals that deplete it.
- The Protocol, which entered into force in 1989, defines a schedule for reducing or eliminating the use of these chemicals.
- Since the adoption of the Montreal Protocol, the Parties also adopted several amendments to that Protocol:
- The London Amendment (1990),
- The Copenhagen Amendment (1992),
- The Montreal Amendment (1997),
- The Beijing Amendment (1999), and
- The Kigali Amendment (2016).
Kigali Amendment to Montreal Protocol
- On October 15, 2016, 197 countries adopted an amendment to phase down HFCs under the Montreal Protocol in Kigali, Rwanda.
- The Kigali Amendment aims for the phase-down of hydrofluorocarbons (HFCs) by cutting their production and consumption.
- The Kigali Amendment to the Montreal Protocol aims to achieve over 80% reduction in HFC consumption by 2047.
- Given their zero impact on the depletion of the ozone layer, HFCs are currently used as replacements for hydro-chlorofluorocarbons (HCFCs) and chloro-fluorocarbons (CFCs) in air conditioning, refrigeration and foam insulation. However, they are powerful greenhouse gases.
- The Kigali Amendment to the Montreal Protocol is legally binding and came into force on January 1, 2019.
- As per the Kigali Amendment to Montreal Protocol:
- Developed countries will reduce HFC consumption beginning in 2019.
- Most developing countries will freeze consumption in 2024.
- Some developing countries, including India, with unique circumstances, will freeze consumption in 2028.
- The plan also provides financing to certain countries to help them transition to climate-friendly alternatives.
- With the Kigali Amendment, the Montreal Protocol has become an even more powerful instrument against global warming.
India’s Efforts to Control Ozone Layer Depletion
India’s efforts to control the ozone layer depletion can be seen as follows:
- India signed the Vienna Convention in 1991 and the Montreal Protocol in 1992.
- India has completely phased out the production and consumption of chlorofluorocarbons, carbon tetrachloride and halons.
- India approved the ratification of the Kigali Amendment in 2021.
- According to this amendment, India has to start phase down by 2028 and cut HFC emission by 15% of 2024-26 levels by the years 2047.
- Carbon tetrachloride, a harmful chemical, is used by some of the largest steel manufacturing units.
- Now, many steel companies use tetrachloroethane, which is less harmful.
- There is a dedicated ozone cell under the Ministry of Environment and Forests, which works in collaboration with UNDP.
Importance of Ozone Layer
- The ozone layer also plays a key role in maintaining the Earth’s temperature balance by influencing the atmospheric circulation patterns.
- The ozone layer helps in preserving biodiversity by protecting both terrestrial and marine ecosystems from UV-induced disruptions.
- Additionally, the ozone layer is vital in reducing UV-related degradation of materials like plastics and paints, prolonging their lifespan.
- Furthermore, the ozone layer supports agricultural productivity by protecting crops from harmful UV radiation, ensuring better yields and food security.
- The ozone layer also protects human health by preventing overexposure to UV rays, which can cause skin cancer, cataracts, and other health issues.
- In this way, the ozone layer plays a crucial role in sustaining life and the environment on Earth.
Conclusion
Ozone Layer Depletion remains a significant environmental issue with complex implications for health, ecosystems, and climate. The global efforts to address this challenge through treaties like the Montreal Protocol have demonstrated that coordinated international action can lead to meaningful progress. Continued vigilance and adherence to these agreements are essential to ensuring the long-term recovery of the ozone layer depletion and protecting the planet’s health.
Polar Stratospheric Clouds (PSCs)
- Polar Stratospheric Clouds are wave clouds formed beyond 60° N and 60° S latitude at an altitude of 15 to 25 km.
- They form at temperatures of around minus 85°C, colder than average lower stratospheric temperatures, and are comprised of ice particles ~10µm across.
- They are also known as nacreous clouds from nacre, or mother of pearl, due to their iridescence.
Role of Polar Stratospheric Clouds in Ozone Depletion
- Polar Stratospheric Clouds (PSCs) play a central role in the formation of the ozone hole in the Antarctic and Arctic.
- They contribute to Ozone Layer Depletion in the following two ways:
- Their surfaces act as catalysts that convert more benign forms of man-made chlorine into active free radicals (for example, ClO, chlorine monoxide).
- During the return of spring sunlight, these radicals destroy many ozone layer molecules in a series of chain reactions.
- Moreover, the cloud formation is doubly harmful because it also removes gaseous nitric acid from the stratosphere which would otherwise combine with ClO to form less reactive forms of chlorine.
Ozone Depletion in Antarctica and Ozone Hole
- A phenomenon of seasonal thinning of the ozone layer occurs in Antarctica, which is known as the “ozone hole”.
- The term “ozone hole” refers to a large and rapid decrease in the abundance of ozone molecules, not the complete absence of them.
- Ozone layer depletion forms because of the special weather conditions and nowhere else.
- The very cold temperatures of the Antarctic stratosphere create polar stratospheric clouds, which allow chlorine and bromine reactions to produce the ozone hole in Antarctic springtime (August-November).
- Peak depletion occurs in October when the ozone layer is often completely destroyed over a range of altitudes.
- The artificial release of ozone-depleting substances, especially enhanced chlorine from anthropogenic chlorofluorocarbons, has aggravated the severity of this phenomenon.
Ozone Depletion in Arctic
- Since the 1990s, significant depletion in the ozone layer has been seen in the Arctic regions.
- However, the maximum depletion is generally less severe than that observed in the Antarctic and is more variable from year to year.
- Although the same basic chemical mechanisms operate in both hemispheres, Arctic ozone layer depletion has not been as marked as over the Antarctic for two reasons:
- The stratospheric temperatures are seldom below –80°C due to frequent exchange of air masses with the mid-latitudes
- The Arctic air vortex usually dissipates in late winter before sunlight returns to initiate ozone layer destruction.
- The differences between the two regions result, in part, from the larger land mass in the northern hemisphere, which causes more activity in the atmosphere.
Good Ozone and Bad Ozone
Ground Level Ozone or Bad Ozone
- The ozone layer found near the earth’s surface in the troposphere is harmful to life (causes breathing issues in humans) and plants (damages crops and plants.) and hence is called Bad Ozone.
- Urban smog comprises the Bad Ozone.
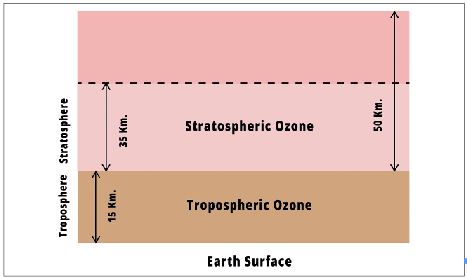
Stratospheric Ozone or Good Ozone
- The good ozone is found in the stratosphere, which also hosts the ozone layer.
- The ozone layer is called good as it absorbs the sun’s harmful ultraviolet radiation.
Frequently Asked Questions (FAQs)
What is Ozone Layer?
The ozone layer is a protective shield in the Earth’s stratosphere containing a high concentration of ozone (O₃) molecules. Ozone layer absorbs most of the Sun’s harmful ultraviolet (UV) radiation, preventing it from reaching the Earth’s surface.
What is Ozone Layer Depletion?
Ozone layer depletion refers to the thinning or reducing of ozone molecules in the stratosphere, mainly due to human-made chemicals like chlorofluorocarbons (CFCs) and halons, which increase UV radiation reaching the Earth.
How is the Ozone Layer Formed?
The ozone layer is formed when ultraviolet (UV) radiation from the Sun strikes oxygen molecules (O₂), causing them to split into individual oxygen atoms. These atoms then combine with other O₂ molecules to form ozone (O₃).
What causes Ozone Layer Depletion?
Ozone Layer Depletion is primarily caused by human-made chemicals like CFCs, halons, and other ozone-depleting substances (ODS). When these chemicals are released into the atmosphere, they break down ozone molecules, reducing the ozone layer’s ability to block harmful UV radiation and cause ozone layer depletion.
How can we protect the Ozone Layer?
We can protect the ozone layer by reducing the use of ozone-depleting substances, promoting eco-friendly products, adhering to international agreements like the Montreal Protocol, and raising awareness about the importance of ozone conservation.






