Syllabus: GS3/ Economy
Context
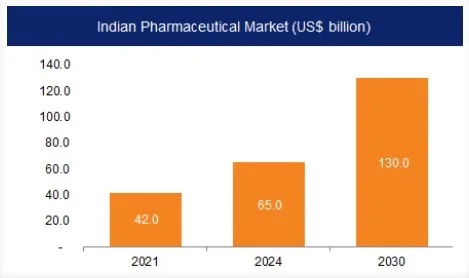
- Union Minister of State for Commerce and Industry, has stressed that the country’s pharmaceutical sector will touch the 130 billion US dollar mark by 2030.
Pharmaceutical Sector of India
- The pharmaceutical industry in India is currently valued at $50 Bn.
- Major segments of industry include generic drugs, OTC medicines, bulk drugs, vaccines, contract research & manufacturing, biosimilars and biologics.
- The Pharmaceutical industry in India is the third largest in the world in terms of volume and 14th largest in terms of value.
- The Pharma sector currently contributes to around 1.72% of the country’s GDP.
- India is the 3rd largest producer of API accounting for an 8% share of the Global API Industry.
Achievements of Pharmaceutical Sector of India
- India accounts for 60% of global vaccine production, contributing up to 70% of the WHO demand for Diphtheria, Tetanus and Pertussis (DPT) and Bacillus Calmette–Guérin (BCG) vaccines, and 90% of the WHO demand for the measles vaccine.
- India supplies over 50% of Africa’s requirement for generics, ~40% of generic demand in the US and ~25% of all medicine in the UK.
- The cumulative FDI equity inflow in the Drugs and Pharmaceuticals industry is US$ 22.52 billion during the period 2000-2024, almost 3.4% of the total inflow received across sectors.
- The nation is the largest provider of generic medicines globally, occupying a 20% share in global supply by volume, and is the leading vaccine manufacturer globally.
- India is known as the “pharmacy of the world” due to the low cost and high quality of its medicines.
Challenges for Pharmaceutical Sector of India
- Intellectual Property (IP) Protection: India’s patent laws, especially concerning compulsory licensing and Section 3(d) of the Indian Patent Act, have led to frequent disputes with multinational corporations.
- Dependence on Imports: APIs and Key Starting Materials (KSMs) import dependence exposes the industry to vulnerabilities related to supply chain disruptions and price fluctuations.
- Skilled Human Resource: Indian pharmaceutical industry requires a highly skilled workforce to drive research and development, manage operations, and ensure quality control.
- Failing the quality tests: The country’s pharma industry has largely been in denial over quality-related concerns expressed by national and international observers.
- According to a Central Drugs Standard Control Organization (CDSCO) survey in 2014-2016, about five per cent of Indian drugs, several of them manufactured by large pharma companies, failed the quality test.
Government initiatives
- The Production Linked Incentive (PLI) scheme for pharmaceuticals is being implemented with a total outlay of the Rs. 15,000 crore (US$ 2.04 billion) spanning from 2020-21 to 2028-29, to boost India’s manufacturing capacity, elevate investment, and diversify product offerings in the sector.
- Pradhan Mantri Bhartiya Janaushadhi Pariyojana (PMBJP): quality generic medicines are made available at affordable prices to all through dedicated outlets known as Pradhan Mantri Bhartiya Janaushadhi Kendras (PMBJK).
- Strengthening of Pharmaceutical Industry (SPI): The scheme is implemented for the period from FY 21-22 to FY 25-26.
- It aims to provide support to existing Pharma clusters and MSMEs across the country to improve their productivity, quality and sustainability to strengthen the existing infrastructure facilities in the Pharma MSME clusters.
- Scheme for Promotion of Bulk Drug Parks: The objective is to provide world class common infrastructure facilities to units located in the parks which will help in significantly bringing down the manufacturing cost of bulk drugs and thereby make India self-reliant.
- 100% Foreign Direct Investment (FDI) in the pharmaceutical sector is allowed under the automatic route for greenfield pharmaceuticals.
- 100% FDI in the pharmaceutical sector is allowed in brownfield pharmaceuticals; wherein 74% is allowed under the automatic route and thereafter through the government approval route.
Way Ahead
- The pharmaceutical industry in India is a significant part of the nation’s foreign trade and offers lucrative potential for investors.
- Speedy introduction of generic drugs into the market has remained in focus and is expected to benefit the Indian pharmaceutical companies.
- In addition, the thrust on rural health programmes, lifesaving drugs and preventive vaccines also augurs well for the pharmaceutical companies.
Source: AIR
Previous article
U.P. Digital Media Policy, 2024